| User Service > Work for Health | Technical support>Technology platform>Work for Health |
Currently, the prevention and control of COVID-19 over the world is still severe. As the first step of nucleic acid detection, the importance of specimen collection and preservation is undoubted. If there is any problem with the specimen collection, the result would be invalid even the following work is done well. Choosing the right Virus transport solution can greatly improves the accuracy of the test result.

1. Have you chosen the right virus transport solution?
The unique non-guanidinium patented formula from Dewei could minimize the risk of "false negative" results.
Almost all virus transport medium on the market now preserves the activated virus, which has a high risk of infection in sampling, transportation, and testing.
The Front-line epidemic prevention workers need preservation solution that can directly inactivate the virus. It should be considered whether the medium can stabilize the integrity of the virus RNA and avoid degradation before testing, which will cause "false negative" results.
We compared the preservation effects of guanidinium medium from other brands and non-guanidinium solution from Dewei at 37°C on avian coronavirus. It was found that the preservation effect of the guanidinium preservation medium is very unsatisfactory!
After 1-7 days of storage under the conditions of 37 ℃, the virus RNA preservation efficiency is basically constant at 100% by using Dewei Virus transport solution. While using the other two guanidinium component medium, the virus RNA preservation efficiency gradually decreases. It was reduced to less than 20% after 7 days, indicating RNA degradation over time.
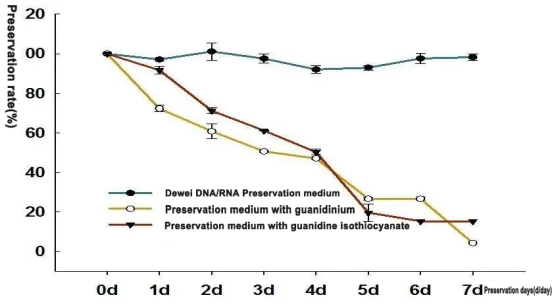
Conclusion: Guanidinium (include guanidine isothiocyanate or guanidine hydrochloride,etc), as a common protein denaturant, is normally used for lysing cell in nucleic acid extraction or inactivating virus. But the medium with guanidinium can not preserve nucleic acid under normal room temperature which easily makes specimen degraded. Therefore, medium with guanidinium is unsuitable for Sars-CoV-2 preservation. It’s strongly suggested to use the non-guanidinium virus preservation solution.
Here, we appeal the worker related to COVID-19 testing to notice about below points when choosing the virus preservation solution to nucleic acid testing only, but not to cultivate virus.
1. Use inactivated virus preservation solution
It’s unnecessary to preserve living virus as long as the intact and stable nucleic acid from virus can be preserved for the test, as currently the purpose of nucleic acid test is to detect the RNA from virus in the samples. Therefore, when in the situation of fighting with COVID-19, inactivated virus during sampling is a better way to preserve samples. In the market, some manufacturers also have inactivated virus preservation medium, but most of the inactivated component is guanidinium, as it was mentioned, medium with guanidinium component can not preserve nucleic acid under normal room temperature. It’s easily degrade virus in the sample and not suitable for preserving Sars-CoV-2 samples. Hence, We recommend Dewei Medical preservation solution with non-guanidinium protein denaturant component, which can inactivate the virus and make sure the viral nucleic acid is not degraded when stored under room temperature.
2. Use the virus preservation solution that can be kept under room temperature
Because the RNA is not stable, common virus transport medium with samples should be preserved in 4℃ condition and delivered to laboratories within 48 hours. If the transport temperature is not standard, the nucleic acid of virus could be degraded, such causes a test result of “false negative”! While, if the sample was kept in Dewei’s non-guanidinium preservation solution that can preserve sample under room temperature, then it can solve the problem of delivery cost and increase the accuracy of testing result.
3. Use virus preservation solution that could protects sample by high temperature inactivation effect
The related instruction of National Health Commission of the People’s Republic of China request that the sample need to be inactivated by high temperature(heat it in 56℃ for 30 min) before extracting virus nucleic, while the institute of Beijing Center for Diseases Prevention and Control and Beijing Research Center of Preventive Medicine publish a thesis on Clinical Chemistry which shows that high-temperature inactivation would degrade the virus nucleic then decrease the virus nucleic detection rate, which is one of the reasons of excessive false negative rate of COVID-19 detection. The experiment shows that high-temperature inactivation has no obvious effect to virus nucleic completeness when use Dewei Medical virus preservation solution to preserve the sample.
Below chart is the data that use Dewei Medical’s virus transport solution to preserve Avian coronavirus, dilute five gradients to extract and test. Compare CT value of normal temperature inactivation with high-temperature inactivation.
|
Gradient |
Normal Temperature Inactivation |
High-Temperature Inactivation in 60℃ for 30 min | ||
|
10-1 |
23.08 |
23.29 |
23.26 |
23.22 |
|
10-2 |
27.26 |
27.43 |
27.32 |
27.48 |
|
10-3 |
32.81 |
32.65 |
32.24 |
32.37 |
|
10-4 |
35.84 |
35.69 |
35.93 |
35.72 |
|
10-5 |
39.65 |
39.79 |
39.83 |
39.78 |
Dewei Medical advise to use preservation solution which is inactivated virus, suitable solution volume, transporting in room temperature and protecting virus to avoid effect from high temperature inactivation when nasal or oral sampling.
Dewei Medical exploits leakproof preservation tube which has unique design of reverse lock. To distinguish traditional tubes which cannot placed upright during transportation, it would easily remaining sample liquid on the nozzle and spill liquid to hands after opening the cover, at the meantime prone to produce aerosol pollution.
Leakproof design without rubber aprons can prevent leaking when repeat the operation of opening the cover, such ensures the safety of sampling operator, also avoids the aerosol and sample leakage caused by the bounce of the rubber aprons.


DEWEI MEDICAL EQUIPMENT CO.,LTD | National Service Hotline: +86 757 25516612 Note: Any third party or instrument name involed in the website, it is only for describing Dewei's Product uses. Copyright: DEWEI MEDICAL EQUIPMENT CO.,LTD